The Knot Series #2: Unraveling the Complexities of DNA and Protein Folding
An intro to understanding how knot theory is applied in DNA topology and protein visualization
MATHBIOLOGYVISUALIZATION
Sunskritha R Shivaprasad
9/29/20242 min read
We now know that knots aren’t completely unrelated to Biology (click here to read my previous blog). The complex folding and twisting of DNA and proteins are similar to mathematical knots that knot themselves into the functional units of life.
Proteins are long chains of amino acids connected through polypeptide bonds, while DNA comprises sequences of nucleotides. These folds are crucial because they determine the molecules’ specific biological function. For example, the double-stranded helix structure of DNA makes the storage and transmission of genetic information possible. And as discussed earlier, proteins fold into secondary, tertiary or even quartentary structures that act as enzymes, structural components, or signaling molecules. These folds are driven by certain intra-molecular forces like hydrogen bonds, disulfide bridges, and other electrostatic interactions. The topological properties of these chains can give scientists important insights on the behaviour of these molecules.
Unraveling the Complexities of DNA and Protein Folding
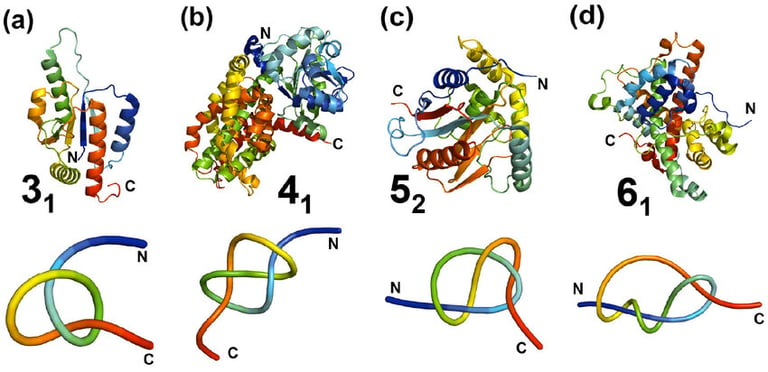
Structures of knotted proteins containing four different types of knots in the polypeptide backbone. (a) a trefoil-knotted (3₁) methyltransferase from E. coli, (b) E. coli class II ketol-acid reductoisomerase, containing a figure-eight (4₁) knot, (c) Human ubiquitin carboxy-terminal hydrolase L1, containing a knot with five crossings (5₂), (d) α-haloacid dehalogenase, containing a stevedore (6₁) knot; Top panel: ribbon diagrams of polypeptide chains colored from blue (N-terminus) to red (C-terminus). Lower panel: simplified views of the knots
After James Wang’s discovery in 1966, that supercoiled DNA relaxes in the presence of DNA topoisomerase, scientists began questioning how DNA topology is crucial in understanding genetic processes.
However, when scientists tried to apply traditional knot theory to proteins, they encountered a problem: most proteins fell into the simplest category, the unknot, meaning they didn’t appear to form any knots at all. This presented a challenge because proteins are known to create complex structures that are functionally relevant.
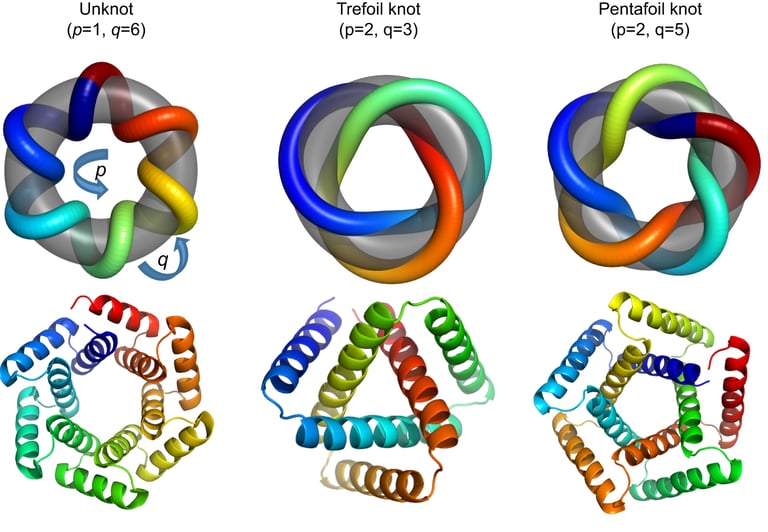
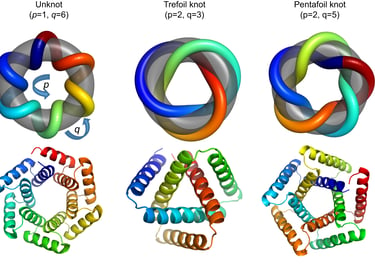
Tandem repeat proteins
Beyond Standard Knot Theory
In order to get around these restrictions, scientists are creating novel knot theories that better represent the intricacy of biological molecules. The addition of singularities—points where the molecule folds back on itself to form intramolecular bonds like hydrogen bonds or disulfide bridges—is one of the major innovations. Standard knot theory ignores these points, despite the fact that they are essential for protein folding and stability.
Scientists can now more realistically model proteins and DNA by incorporating these singularities into knot theory. For instance, they are able to categorize proteins based on the particular bonds and interactions that maintain their structure in addition to their general shape. We can now better understand how proteins fold and how misfolding may contribute to illnesses like Alzheimer's thanks to this novel framework, which is frequently referred to as singular knot theory.
Read my next blog to find out more!
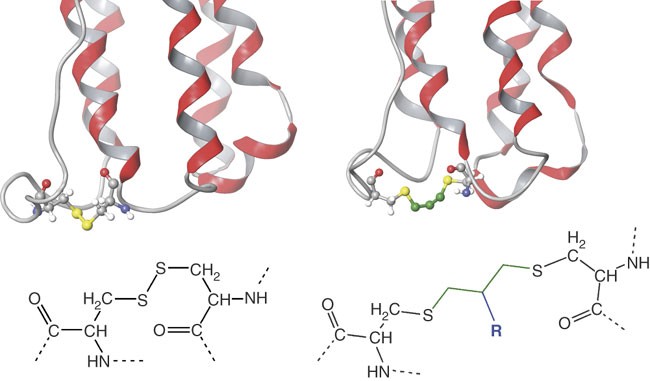
Native (yellow) and 3-carbon-bridged disulfide bond (green) of interferon α-2a.
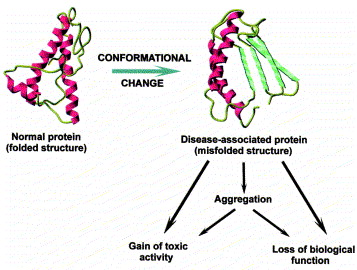
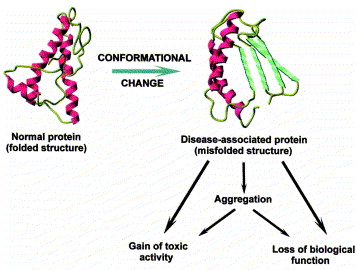
Protein misfolding resulting in Alzheimer's diease

Contacts
sunskrithas19@gmail.com
Blog
© TheArtGene
sunskrithas@theartgene.com
Subscribe to our blog!
Updates bi-weekly every Sunday!
